Zepto Lab Services
Innovative solutions in clinical diagnostics and research — all under one roof
What We Offer
Zepto Life Technology provides two core services: full-spectrum CRO solutions for
research and regulatory needs, and certified diagnostic testing for patients and providers.
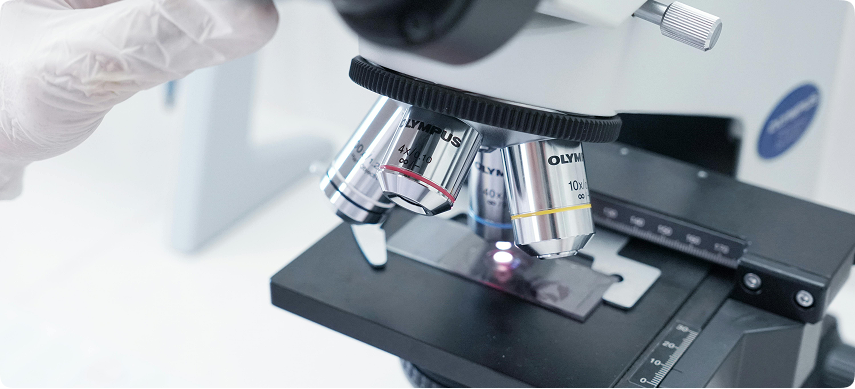
We provide comprehensive Contract Research Organization (CRO) services for preclinical and clinical studies, tailored to support your regulatory submissions and product validation.
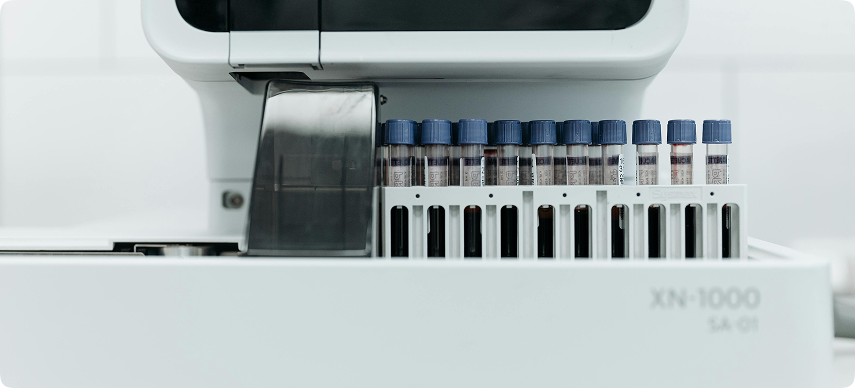
Our CLIA-certified lab delivers accurate, reliable, and fast diagnostic testing services to patients and providers across the country.
CRO Services Overview
Our Contract Research Organization services support every phase of your clinical journey
— from study design and device analysis to regulatory reporting and validation.

Facilities:
Our facility includes modern laboratories equipped for a full range of testing — from routine assays to complex biomarker analysis.
Team Expertise:
Our team brings together decades of lab experience, scientific insight, and regulatory knowledge to guide your product from study to submission.
Key Services:
Analytical testing services Central reference lab Regulatory & submission support Customized reporting
Diagnostic Testing Overview
We offer fast, accurate, and confidential diagnostic testing performed in our CLIA-
certified lab — designed to support individual patients and healthcare providers alike.

Description:
We offer a broad range of high-quality diagnostic services, including respiratory panels and other clinical tests — all processed in our CLIA-certified laboratory.
Patient-Centric:
Trusted by thousands of patients, our lab combines accuracy, confidentiality, and speed for the best testing experience.
Why Zepto?
Learn why Zepto is so many company’s first choice for analytical
testing & central reference lab testing
Extensive IVD development technology expertise and regular implementation of IVD
Our in-house high complexity CLIA certified central laboratory allows for internal lab developed tests (LDT)
Fully customized clinical trial support for study management, biostatistics & data management, and many more
Successful completion of various FDA EUA and 510(k) applications as well as analytical studies and validations
Professional bilingual communication in both English & Mandarin for scientific services and translations
Key Services
Our CRO unit specializes in analytical study testing for a wide variety of product types as well as
our in-house high complexity CLIA certified central reference lab testing.

Analytical Study Testing
Our team of scientists have extensive knowledge in both IVD development as well as IVD submissions. Our expertise in IVDs means that we can offer both product validation as well as product optimization.
Regulatory Strategy and Submission Support Risk Evaluation
Product Adjustment Support Product Validation Market Comparison & Customized Reports for Global Non-U.S. Sale

Central Laboratory
Our in-house high complexity CLIA certified central laboratory allows for customizable testing services as well as lab developed test (LDT) capabilities.
High Complexity CLIA Certified Lab Trained Professional Staff Quick Turnaround and Data Reporting Customized Client Reports Post Authorization Quality Monitoring Plan/Testing Lab Developed Test (LDT) Capabilities
Professional Facilities
Our comprehensive suite of services includes regulatory support and FDA communication and consulting, leveraging extensive expertise in medical writing and operations. We excel in central lab services, analytical performance, and possess expertise in molecular diagnostics, point-of-care testing, over-the-counter testing, rapid lateral flow test, and immunoassay. Our team is proficient in professional communication in both English and Mandarin, providing a unique advantage. We offer Lab Developed Test (LDT) capabilities and flexible options for full or customized clinical trial support, encompassing study management, biostatistics, and data management. Additionally, our services extend to site selection, site monitoring, safety, technical and regulatory writing, as well as Quality Management System (QMS) development and compliance.
Zepto’s CRO team is ready and equipped to support your product submissions every step of the way. Our experiences include FDA Emergency Use Authorization (EUA) & 510(k) submissions as well as submissions for countries outside of the United States.
Zepto’s CRO team is ready and equipped to support your product submissions every step of the way. Our experiences include FDA Emergency Use Authorization (EUA) & 510(k) submissions as well as submissions for countries outside of the United States.
Zepto’s CRO team is ready and equipped to support your product submissions every step of the way. Our experiences include FDA Emergency Use Authorization (EUA) & 510(k) submissions as well as submissions for countries outside of the United States.
Zepto’s CRO team is ready and equipped to support your product submissions every step of the way. Our experiences include FDA Emergency Use Authorization (EUA) & 510(k) submissions as well as submissions for countries outside of the United States.
Our CRO Team’s Expertise

Zepto Life Technology is a provider of analytical studies with a proven track record in supporting FDA (EUA) and 510(k) applications.
- Regulatory Strategy and Submission Support
- Risk Evaluation
- Product Adjustment Support
- Market Comparison & Customized Reports for- Global Non-U.S. Sale
- Product Validation
- Regulatory Strategy and Submission Support

Zepto Life Technology’s onsite high-complexity CLIA and BSL 2+ laboratory is equipped with state-of-the-art instruments.
- High Complexity CLIA Certified Lab
- Trained Professional Staff
- Quick Turnaround and Data Reporting
- Post Authorization Quality Monitoring Plan/Testing
- Customized Client Reports
- High Complexity CLIA Certified Lab
Our Amazing Clients
Here are some of our past clients whom we have helped
on their mission to shape the healthcare landscape!





Want to
talk to us?
Get in touch.
Monday – Friday
09:00 a.m. - 05:00 p.m.
Note: Though we are not open on weekends, we are willing to accommodate special requests with prior notice. For more information, feel free to give us a call or fill out the contact form!
Enter your email and your message and we’ll connect you with the right person!